Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been spreading worldwide and become a serious global health problem by causing the novel coronavirus disease 2019 (COVID-19). However, no licensed preventative vaccines are available for COVID-19 until now. To develop a COVID-19 vaccine with global supply scale, Yang et al. produced a recombinant S-RBD protein using a commercial baculovirus expression system, which has already been applied to produce many other vaccines, including influenza vaccines. Here, Prof. Xinwei Wang and Wenwei Tu highlighted the key points in this study and provide a vision based on it.

In a recent study published in (Nature_https://doi.org/10.1038/s41586-020-2599-8: 2020), Yang et al. developed a COVID-19 vaccine with high efficacy to induce neutralizing antibodies targeting the receptor-binding domain (RBD) of spike (S) protein (S-RBD) and prevent SARS-CoV-2 infection in multiple animal models, including non-human primates. This study not only gave new insights for developing COVID-19 vaccine, but also provided a readily applicable strategy to prevent SARS-CoV-2 infection.
SARS-CoV-2 contains four structural proteins, including the nucleocapsid, membrane, envelope and S proteins. Among them, S protein is the most important therapeutic target as it plays pivotal roles in viral attachment, entry and fusion. Currently, most vaccine candidates against COVID-19 are designed to target the S protein to elicit neutralizing antibodies for blocking virus entry. Compared with those targeting the whole S protein, the vaccine candidates targeting the S-RBD are more scientifically feasible, because they can avoid inducing the non-neutralizing antibodies targeting the non-RBD regions, which may cause side effects like antigen-dependent enhancement. In addition, the S-RBD region has multiple conformational neutralizing epitopes and is relatively conserved, making it as one of the critical targets for vaccine development against COVID-19.
In addition, this vaccine was developed with a commercial baculovirus expression system considering possible global supply scale and was developed by co-precipitating alum and the S-RBD protein, providing higher applicability and safety. In later studies, the vaccine candidate was found safe and could effectively induce neutralizing antibodies to protect animals, including non-human primates, from live SARS-CoV-2 infection. As this vaccine candidate can be produced by a commercially feasible system at a massive scale, it has a great potential to fight against the global pandemic of COVID-19 and deserves to be evaluated in clinical trials as soon as possible (Fig. 1).
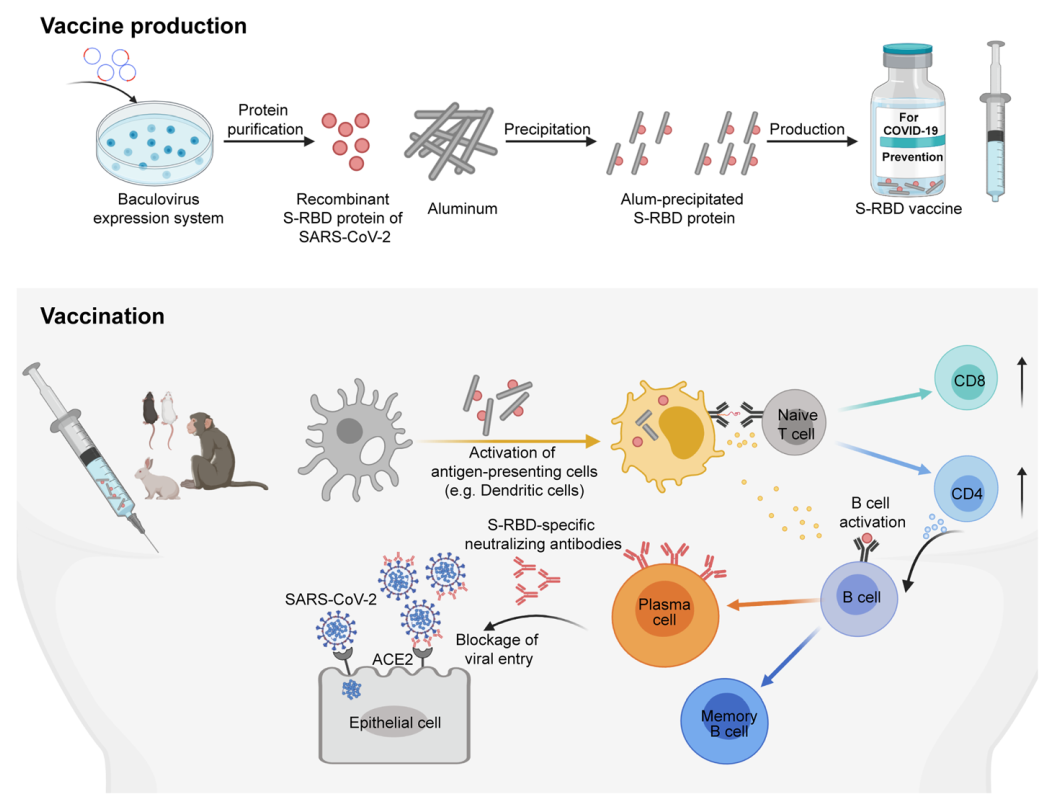
Fig. 1 Scheme of generating and evaluating the vaccine candidate targeting the spike protein receptor binding domain (S-RBD) of SARS-CoV-2
Article Access: https://link.springer.com/article/10.1186/s43556-020-00008-x
Website for Molecular Biomedicine: https://www.springer.com/journal/43556
Looking forward to your contributions