Toll-like receptors (TLRs) play a vital role in both innate and adaptive immune responses, which means they are also indispensable in tumorigenesis. Up to now, some TLRs, such as TLR8, TLR4, TLR7 and TLR9 have been demonstrated as cancer drug targets. In this study, researchers developed a novel TLR8 agonist for cancer immunotherapy, which showed significant potentiality as a monotherapy in treating cancer patients and some other diseases.

Toll-like receptors (TLRs) are a family of proteins that recognize pathogen associated molecular patterns (PAMPs). Their primary function is to activate innate immune responses while also involved in facilitating adaptive immune responses. Different TLRs exert distinct functions by activating varied immune cascades. Several TLRs are being pursued as cancer drug targets. In the present study, the authors discovered a novel, highly potent and selective small molecule TLR8 agonist -- DN052. DN052 exhibited strong in vitro cellular activity with EC50 at 6.7 nM and was highly selective for TLR8 over other TLRs including TLR4, 7 and 9 (Fig. 1).
Furthermore, DN052 displayed excellent in vitro ADMET and in vivo PK profiles and potently inhibited tumor growth as a single agent. Moreover, combination of DN052 with the immune checkpoint inhibitor, selected targeted therapeutics or chemotherapeutic drugs further enhanced efficacy of single agents. Mechanistically, treatment with DN052 resulted in strong induction of pro-inflammatory cytokines in ex vivo human PBMC assay and in vivo monkey study. GLP toxicity studies in rats and monkeys demonstrated favorable safety profile. This led to the advancement of DN052 into phase 1 clinical trials.
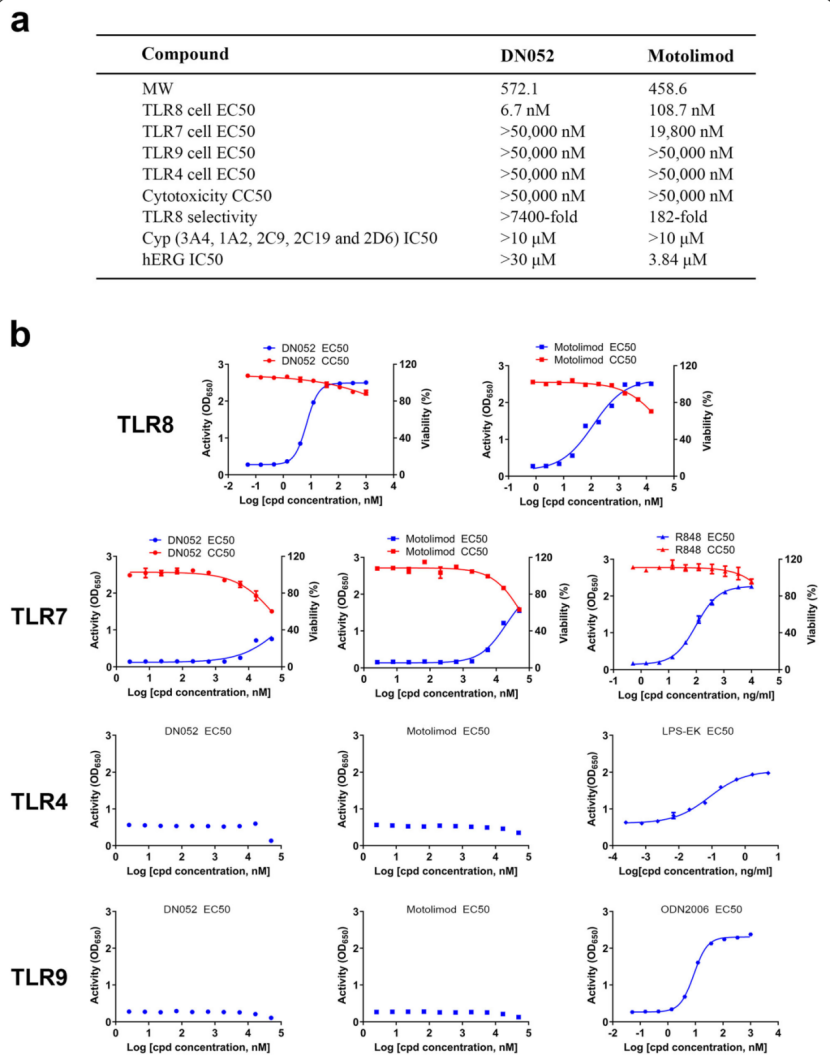
Fig. 1 In vitro profiles of DN052
Article Access: https://link.springer.com/article/10.1186/s43556-020-00007-y
Website for Molecular Biomedicine: https://www.springer.com/journal/43556
Looking forward to your contributions