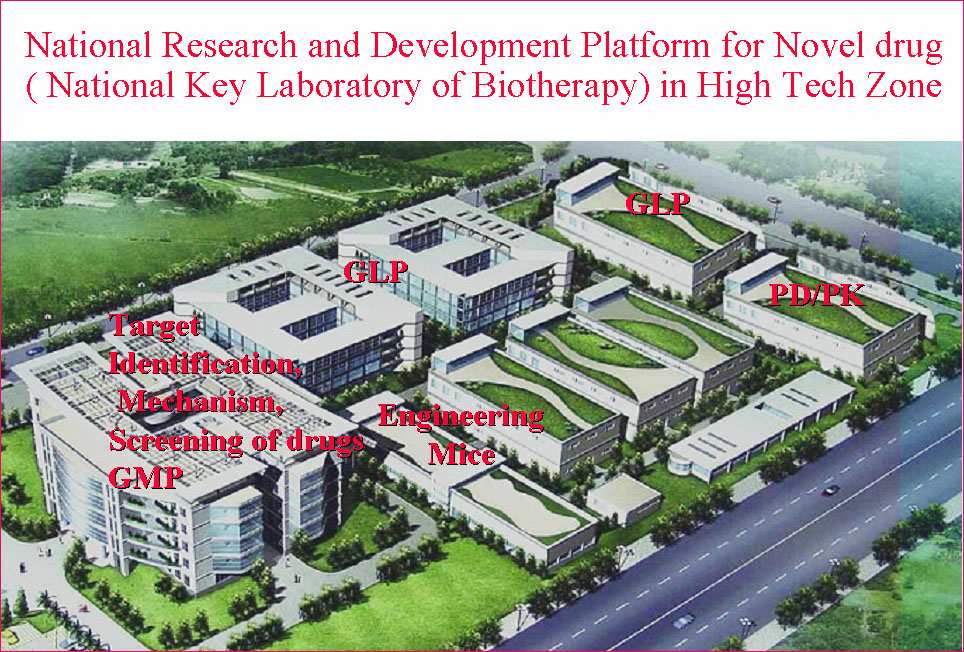
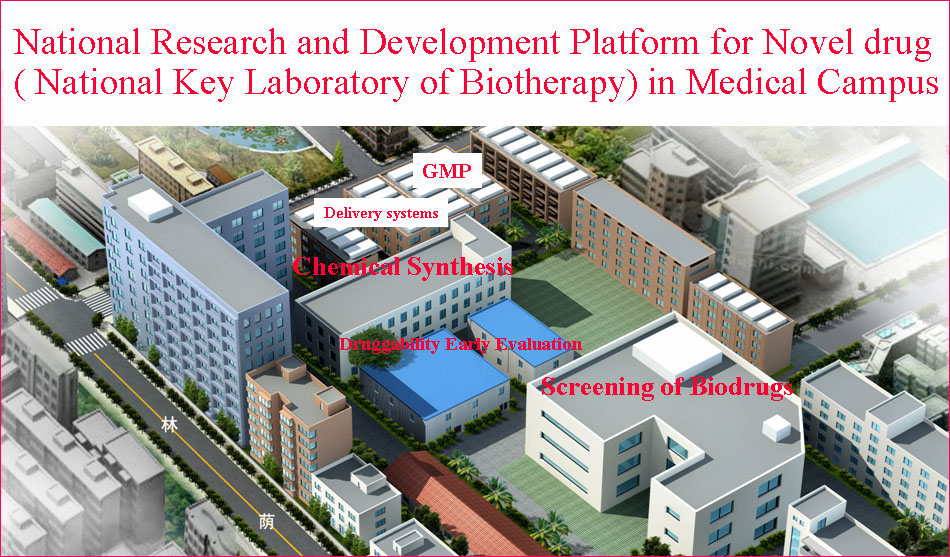
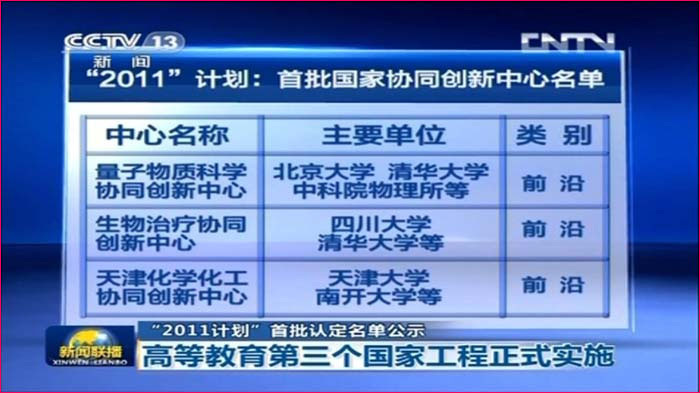
State/National Key Laboratory of Biotherapy (SKLB) was established by the Ministry of Sciences and Technology in China in 2005, and became one of the National Research and Development (R&D) Platforms for Novel Drugs founded by the Ministries of Sciences and Technology as well as Public Health in September 2008. It is also supported by National Collaborative Innovation Program and became the National Collaborative Innovation Center for Biotherapy in April 2013. The Platform is located in the High Tech Zone of Chengdu and the Medical Campus of Sichuan University. The overall space is nearly 70,000 square meters and is still under intensive growth and construction. SKLB also has rich clinical resources of West China Hospital, which has 4,300 beds.
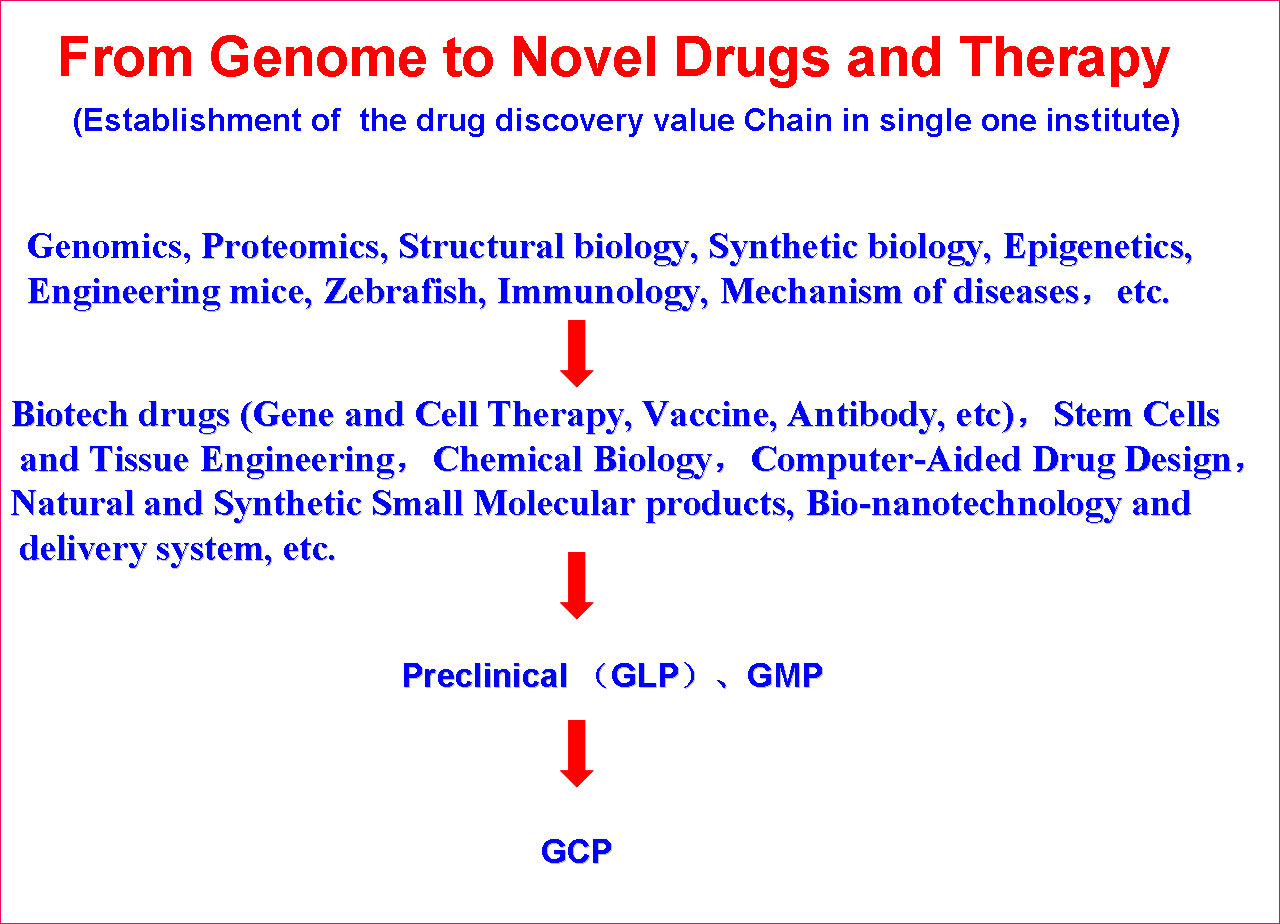
SKLB is becoming a highly-regarded, comprehensive and multidisciplinary research center in China. Through the seamless integration of basic research, preclinical development, translational and clinical medicine, an efficient and fully integrated technology chain for the discovery and development of innovative drug candidates has been established in a single institute. Part of the mission of this Platform is to improve the treatment of major human diseases such as cancer, cardiovascular diseases, obesity, diabetes mellitus, infectious diseases (hepatitis, AIDS, and tuberculosis etc), inflammatory diseases, neurological disease, as well as chronic autoimmune diseases. Focusing on more than 200 important targets, hundreds of projects for basic research and biologic drugs (such as gene and cell therapy, vaccines, and monoclonal antibodies, recombinant proteins, etc), synthetic small molecule drugs and small molecule natural products are ongoing.
The Platform boasts a prominent faculty of nearly 100 well-funded full professors, associate professors and assistant professors, with diverse disciplines such as biotechnology, medicine, immunology, pharmacology, chemistry, formulations, drug delivery, and material sciences. Every year the faculty can obtains competitive grants from the government, including the National High Technology Research and Development Program (“863” Program) or the Major State Basic Research Development Program (“973” program). Over 300 research papers are published in peer-viewed international journals every year, including such top journals as New Engl J Med, Nat Rev Drug Discovery, Dev Cell, Nat Chemical Biol, Nature Med, Mol Cell, Proc Natl Acad Sci USA, Cancer Res, and Lancet Neurol. To date, the Platform has licensed over 50 patents to the commercial sector throughout China. Furthermore, 45 potent candidate drugs thus far have been transferred to over 30 pharmaceutical companies for commercial development.
The Platform also provides both key technological services and integral solutions in R&D for other institutions and companies, including:
(1) Target Identification and Drug Discovery
The Platform has established multiple sub-platforms, such as Proteomics and genomics, structural biology, epigenetics, immunology, molecular cell biology,developmental biology,the mechanism of diseases, biopharmaceuticals R&D, gene and cell therapy, monoclonal antibody and vaccine, stem cell/tissue engineering,engineering mice and zebrafish, drug delivery and nanotechnology, computer-aided drug design, chemical biology,screening and synthesis of small molecular drugs, and the separation and purification of active natural products, especial in the development of novel therapeutic approaches.


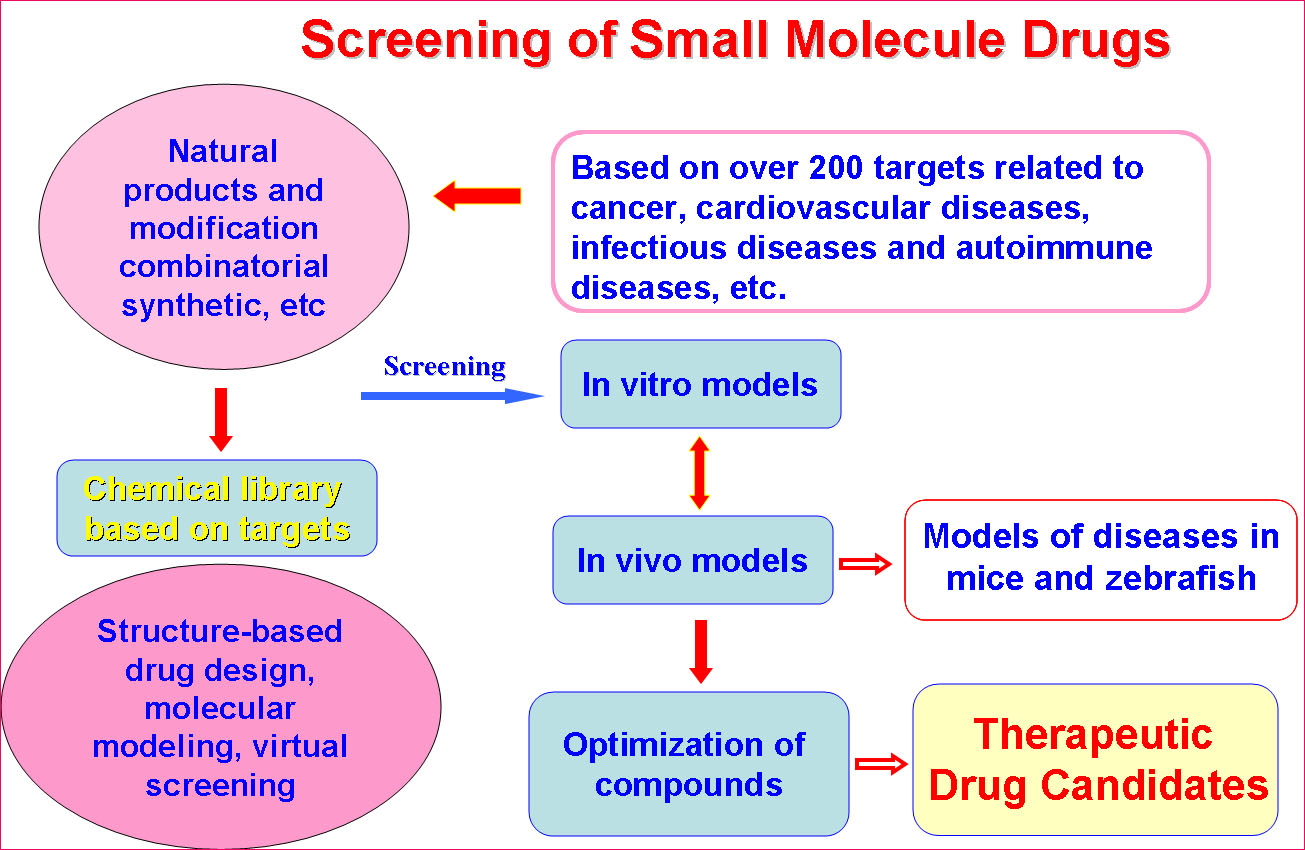
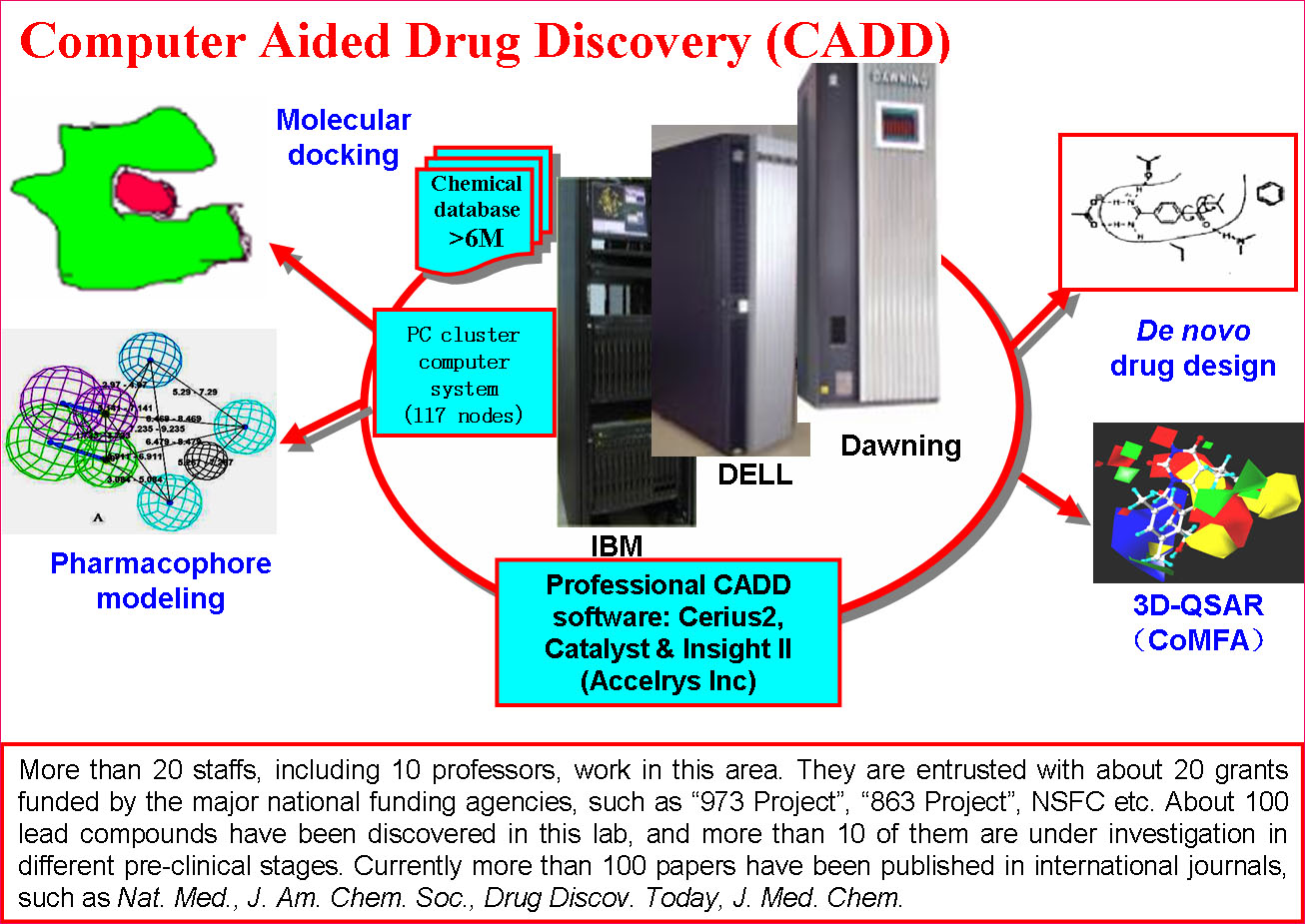

(2) Pilot-Scale Production
Six GMP-level facilities for pilot-scale production of adenoviral vectors, vaccines, DNA plasmids, recombinant proteins and nanoparticles have been established. For example, the pilot-scale production of DNA plasmids provides 5000-8000 doses for early phase clinical trials.

(3) Pharmacodynamics & Pharmacokinetics
Three separate animal facilities can maintain up to 60,000 mice. At full capacity, 300 drug candidates can be evaluated at the same time. To date, there have been over 100 different preclinical disease models established at these facilities.

(4) Pre-Clinical Safety Evaluation
The animal center has currently obtained international“AAALAC”accreditation. The GLP facility can maintain up to 500 monkeys. Various toxicity studies including single dose toxicity, repeated dose toxicity, reproductive and developmental toxicity, genotoxicity, carcinogenicity, immunogencity, and pharmacokinetic/toxicokinetic (PK/TK) analyses are all performed.

(5) Clinical trials
Clinical trials testing gene and cell therapy, cytokines and anti-cytokines, monoclonal antibodies, chemotherapy and targeted small molecule drugs are conducted in the clinical units of West China Hospital with 4300 beds.

(6) Graduate programs
The platform also is an educational center. Every year the platform enrolls about 150 Ph.D and 100 M.S students for different academic programs, as well as nearly 100 M.S. graduate students for pharmaceutical engineering programs.